Design considerations for medical device injection molding
Designing medical devices for injection molding requires extensive understanding, reasoning and planning. Careful consideration of certain aspects throughout the design process will yield favorable results.
There are different aspects to evaluate in order to make certain the actual design meets the desired criteria. Design engineers need to account for part size, impact, material selection, production volume and budgets during the early stages of design. These aspects are major components that drive future decisions of mold designs, components, options and costs.
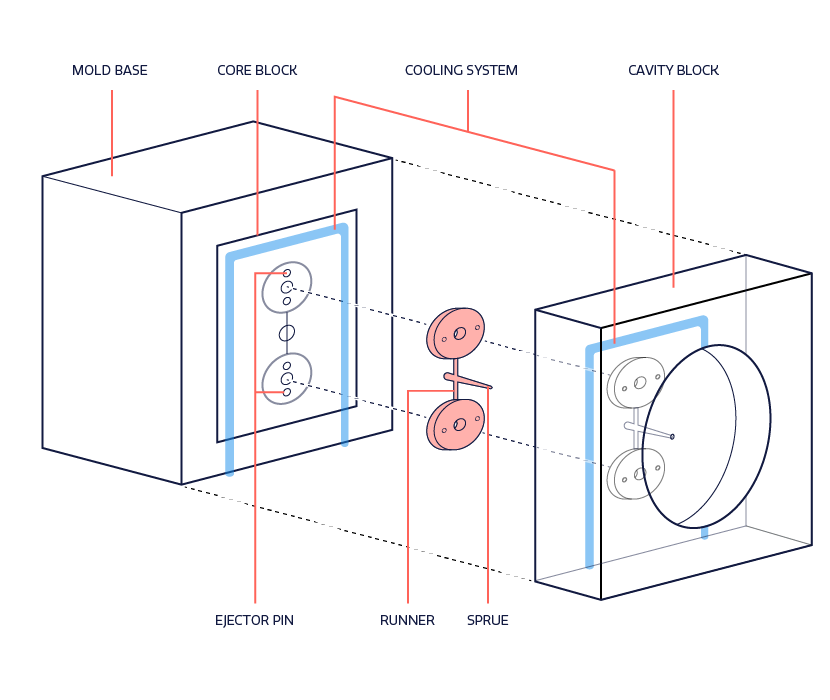
We have compiled major considerations that designers should examine when designing medical devices that rely on plastic injection molding. These considerations, if properly addressed, will reduce overall tool and part costs as well as benefit the manufacturing process by achieving the highest possible quality and reliability.
Considerations during the design of medical devices for injection molding
Draft
The most misunderstood consideration design engineers run into is incorporating proper draft into the part design. Draft is a slight tapering of the part that allows it to eject from the cavity. As the plastic part cools in the tool, it shrinks, which causes it to adhere more firmly to the core of a mold. A draft on the part of just a few degrees reduces this effect. Draft also reduces frictional damage during ejection. A draft of ‘0.5 to 3.0’ degrees should be considered depending on texture.
Shrinkage allowance
Each plastic material shrinks as it cools. Fortunately, plastics sometimes come with their own shrinkage data to be accounted for during design, although it can still be difficult to estimate the shrink value of some materials. Shrink is incorporated into the mold design and thus designers must be aware of its impact, especially for materials that do not have a well-defined one.
Wall thickness
Injection molding does not work well with too thick or too thin walls. In particular, wall thickness can make gate placement challenging. It is difficult to gate on thin wall sections. Further, plastic material does not flow effectively from thin to thick sections of the cavity. Uniform wall thicknesses provide for the most flexibility in deciding on gate location and can reduce the potential for warping, sink and other defects.
Parts of the mold that need to be accounted for when designing
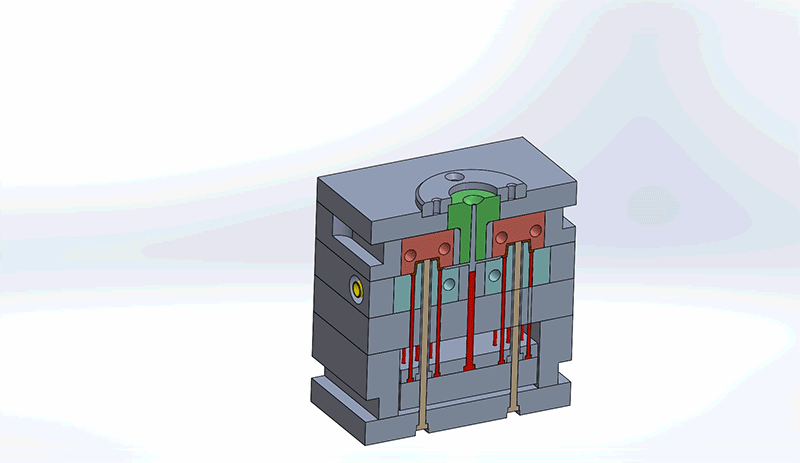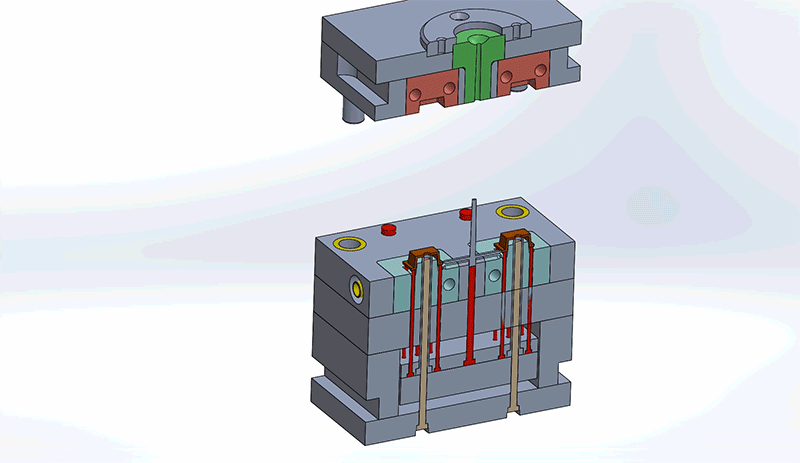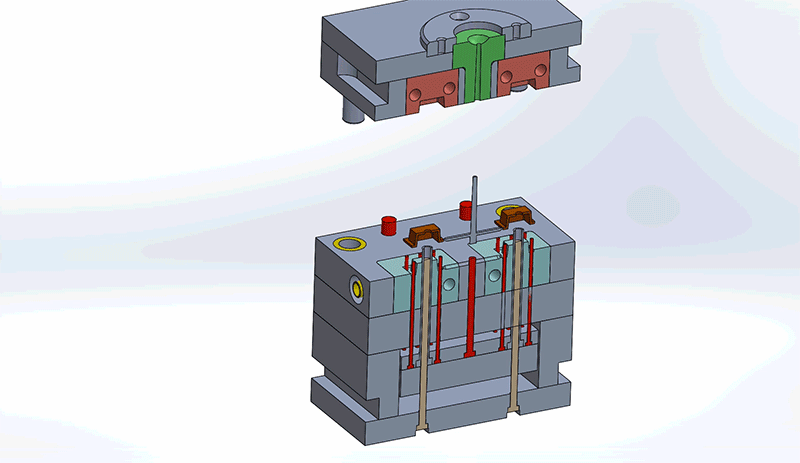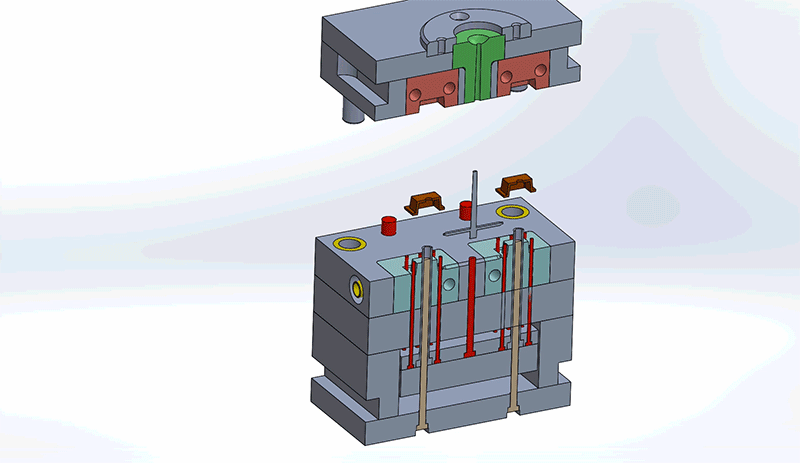
Design engineers must consider all components in the mold when reviewing designs for best Design for Manufacturability (DFM) practices. Understanding how the mold components impact design and the overall injection molding process will improve the general quality of the final medical device.
Gates
Gates are the entry points for molten plastic to flow into the cavity of the mold. There are many types of gates – edge, ring, diaphragm, sub-gate, direct sprue, etc. – that are used based on the shape of the device. The sprue is the initial gate, where plastic is received from the injection molding machine. It can also run as a hot tip gate, which is used with hot runners. Hot tips gates eliminate the “runner” for the part, allowing for faster cycle times and reducing the amount of material used in molding the part. It should be noted that sprues can be removed (generally to save material costs) with the use of hot runner systems. It can also be known as a hot tip gate, which is used with hot runners.
Gate location is important to design so plastic resin flows efficiently and fills the mold. Consider locating gates at meaningful cross sections. This will minimize voids and sink while ensuring the cavity if filling properly. Further, place gates away from cores and pins in order to mitigate obstructions in the flow path. Gate locations should always improve efficiency without negatively impacting the functionality or aesthetic of the part. In fact, there is software available to help determine optimal gate locations.
Runners
Runners are channels in the mold that carry plastic from the sprue to the gates. There are two types of runners: hot and cold runner systems. Hot runners are more expensive, but life cycle costs are considerably lower due to reduced plastic waste, shorter cycle times and design flexibility. When material costs are particularly expensive and part volume demands are high, hot runner systems are ideal. Cold runner systems use unheated channels. A mold with cold runners will need a large enough shot of molten plastic to fill the cavity and runners. Cold runner systems are ideal for lower product volumes and less expensive materials.
Ejector system
Every molded part requires a way to be ejected without causing damage to the part or the mold. Proper draft will facilitate the ejection process, but each mold still needs a system for ejection. There are many kinds of ejector systems, from pin and sleeve ejections to blade and air ejections. Understanding ejection systems will influence design determinations to find a balance that promotes the integrity of the part and mold. In fact, ejector systems leave a “mark” on locations that need to be considered for high cosmetic parts.
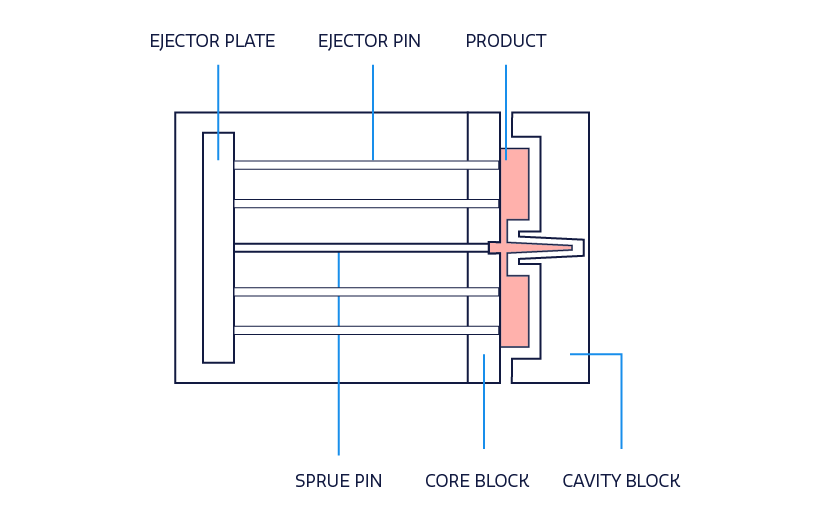
Cavity and core plate
The cavity and core plate, or block as it is also called (see image), is the section of the mold to be filled with the plastic resin and accounts for the external shape of the device. The cavity and core combine to form the part geometry based on the parting line. When designing the cavity and core plate, it is important to incorporate the draft and account for decorative and textured components. However, note that the decorative and textured components may be better accounted during the finishing stage of the manufacturing process.
Cooling circuit
Essentially, a mold is a complex heat exchanger. The mold must be hot enough to effectively enhance the molding cycle and be designed to cool as quickly as possible. The cooling circuit removes the heat by running water through hollowed channels in the mold. This expedites cooling to help meet the cycle times necessary for production.
Most companies reach out to EirMed once their designs are completely finished and ask our engineers to adjust the designs for better DFM practices. We strongly recommend coming to EirMed when you begin designing your device. Our years of experience in medical device injection molding can influence the design to improve efficiency, ensure part success and target the lowest overall cost. EirMed will be able to assist with DFM at any stage of the design of your device, preferably the earlier the better.
Medical device design for injection molding
Designing medical devices requires a thorough understanding of the design elements and the manufacturing factors that influence design.





Leave a Reply
Want to join the discussion?Feel free to contribute!